Comprehensive Solutions
Customized clinical trial solutions bring innovative therapies to market efficiently, reliably, and with global regulatory support.
Provide end-to-end support across all clinical trial phases—streamlining protocol design, data collection, CSR submission and regulatory compliance.

Vaccine Clinical Trials
Oncology Clinical Trial
Scalable Solutions






We develop adaptive, patient-centric trial designs tailored to complex oncology indications.
Our team integrates biomarker strategies, protocol optimization, and regulatory foresight to enhance trial success and reduce timelines.
From site selection to patient enrollment and data capture, we ensure efficient trial execution.
Leveraging a global network of oncology-specialized sites, we maintain high-quality standards and rapid startup timelines.
Parkinson's Disease
Data-Driven Trial Optimization
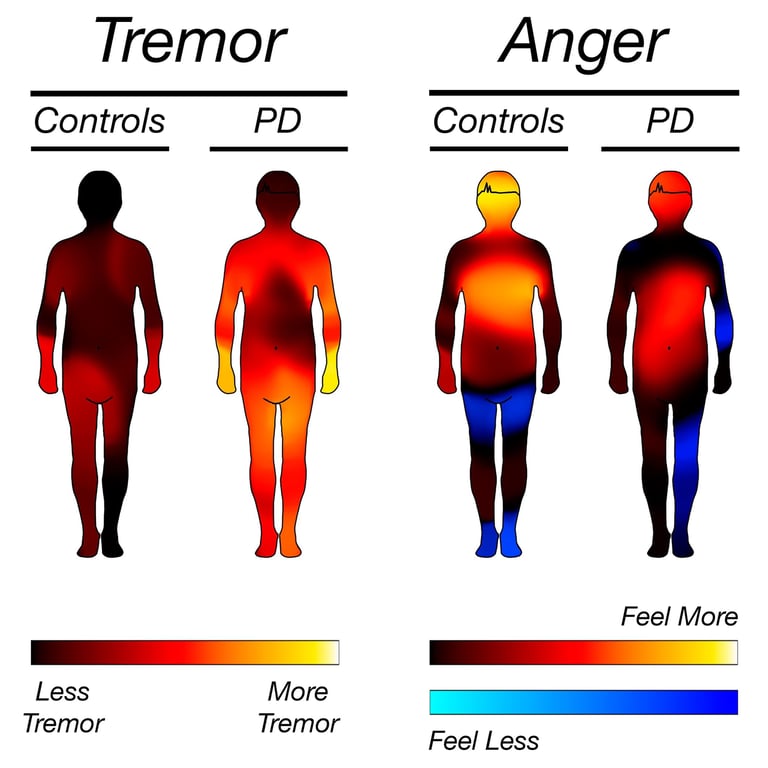
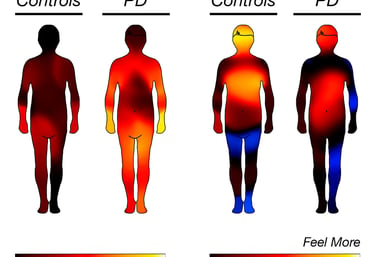
Patient-centric approach ensures higher retention and real-world relevance across all trial phases.
Integrate biomarkers, digital endpoints, and adaptive designs to maximize outcome precision.
Craniopharyngioma
Therapeutic Expertise Across Indications
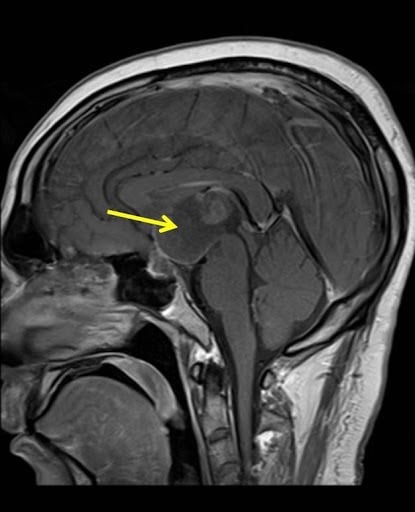
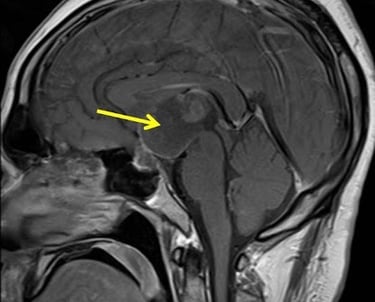
Integrate neurosurgery, endocrinology, radiotherapy, and neuropsychology to ensure comprehensive care and trial support.
Targeted clinical protocols for craniopharyngioma, addressing its unique location, low incidence, and complex impact on neuroendocrine function and cognition.


Comprehensive Namtso research solutions for craniopharyngioma, a rare and complex brain tumor.
Other Therapeutic Areas
Diabetes




Bio-informatics
Eye Care
Our services span patient recruitment, site management, and outcomes tracking to ensure impactful and regulatory-ready results.
